A common consequence of cartilage defects is swelling, pain, or immobility of the knee joint, which in the long term can lead to a reduction in weight-bearing capacity and even mobility. However, there are various treatment options for localized cartilage defects. One of them is the autologous matrix-associated chondrocyte implantation (ACI).
ACI has been around since 1983 and has been steadily gaining popularity. Most often it is used to treat cartilage defects in the knee, the ankle or the hip. It is a two-stage operative procedure. Since the introduction of ACI an open approach was necessary, however the use of spheroids make minimally invasive or even arthroscopically application feasible.
Within the method of ACI, CO.DON has developed a special technology – the spheroid technology: Three-dimensional cartilage cell implants – the spheroids – consisting of healthy cartilage cells and extracellular matrix that are produced ex vivo in a laboratory and implanted back into the cartilage defect to form new cartilage tissue.
Derived from this technology is the name of the product: SPHEROX. Its special feature: Spherox is 100% autologous. The product contains nothing foreign to the patient’s body, only the patient’s own cartilage cells and extracellular matrix. Spherox is classified as Advanced Therapy Medicinal Product (ATMP).
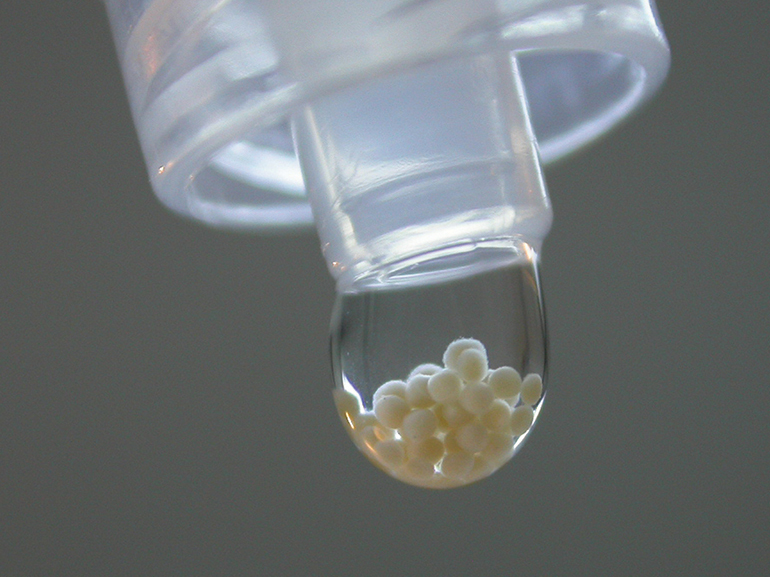
Spheroids are spherical cartilage cell implants made from human chondrocytes and extracellular matrix to treat cartilage defects in the (knee) joint. They are manufactured individually for each patient. Over a period of about 6 – 8 weeks they are produced in a laboratory and afterwards implanted as Spherox into the cartilage defect.
The spheroids are applied evenly into the defect. Just a few minutes later, they self-adhere to the subchondral bone. After about 20 minutes, the process is complete and the spheroids adhere firmly.
The animation shows spheroid implantation into the cartilage defect of the knee joint (model enlarged).
During the following weeks and months after the implantation of the spheroids (Spherox), new hyaline-like cartilage gradually forms and integrates into the existing, healthy cartilage. After successful treatment and personalised rehabilitation, the knee can bear weight again.
The animation shows in time laps how new hyaline-like cartilage tissue has formed in the cartilage defect.
ACI is a two-stage operative procedure that can be performed minimally invasive. See how the biopsy and implantation are carried out when treating a cartilage defect in the knee with Spherox.
The video shows the debridement of the defect and the implantation of the spheroids. The spheroids are provided in a pre-filled syringe or an applicator and are applied evenly into the defect ground. The spheroids self-adhere within 20 minutes onto the subchondral bone. No additional cover of the treated area or any fixation of spheroids is needed.
Dr. Arnd Hoburg is knee specialist and experienced user of ACI. He works in the Knee Surgery Section at MedCenter 360° Berlin, Germany. Find out from him how ACI works, what results it can achieve and for whom it is suitable.

The video explains which conditions should be considered for an ACI.

Dr. Hoburg explains the method of ACI and how the treatment is carried out.

It is described what procedures are necessary for ACI, how the procedure looks like, what steps are necessary after ACI, and how long the rehabilitation takes.

The video describes what results can be expected from ACI and how the patient feels after the treatment.
Learn more about the scientific background of ACI with spheroid technology and find an overview of clinical studies.

Artificial knee joint or cartilage cell therapy? Christian Florkiw-Jenkner talks about the treatment of his cartilage defect with SPHEROX and how it helped him to play sports again without pain.

Klara Tomsova cannot imagine her life without sport. Severe pain in her knee, forces her to give it up. Until a treatment with SPHEROX enabled her to return to an active life.
Spherox is EU-approved. Please find below the Summary of Product Characteristics for Spherox for the countries the product is currently available in.
The abbreviated prescribing information is based on the English Product Information from EMA. It is also available via the following link: https://www.ema.europa.eu/en/
medicines/human/EPAR/spherox.
Name of the medicinal product: Spherox 10-70 spheroids/cm2 implantation suspension; active ingredient: spheroids of human autologous matrix-associated chondrocytes. Composition of medicinal product: 10-70 spheroids/cm2 defect suspended in isotonic sodium chloride solution. Therapeutic indication: Repair of symptomatic articular cartilage defects of the femoral condyle and the patella of the knee (International Cartilage Regeneration & Joint Preservation Society [ICRS] grade III or IV) with defect sizes up to 10 cm2 in adults and adolescents with closed epiphyseal growth plate in the affected joint. Contraindications: Patients with not fully closed epiphyseal growth plate in the affected joint, primary (generalised) osteoarthritis, advanced osteoarthritis of the affected joint (exceeding grade II according to Kellgren and Lawrence), infection with hepatitis B virus (HBV), hepatitis C virus (HCV) or HIV I/II. Undesirable effects: Information on adverse reactions from clinical trials and a non-interventional study in adolescents as well as from post-marketing experience are available. In general, the adverse reactions in paediatric patients were similar in frequency and type to those seen in adult patients. Adverse reactions associated with Spherox: common adverse reactions: joint effusion, arthralgia, joint swelling, bone-marrow oedema, pain. Uncommon adverse reactions: chondromalacia, joint noise, joint lock, synovial cyst, chondropathy, synovitis, loose body in the joint, gait disturbance, hypertrophy, graft loss. Rare adverse reactions: cellulitis, osteomyelitis, hypersensitivity, osteochondrosis, osteonecrosis, osteophyte formation, arthritis infective, graft delamination, implant site infection, infrapatellar fat pad inflammation. Not known adverse reactions: arthrofibrosis. For further information, refer to Summary of Product Characteristics and Package Leaflet. Warnings: Spherox is an autologous medicinal product and must not be given to any other patient than the donor. Note: Available only on prescription. Pharmaceutical company: CO.DON GmbH, Deutscher Platz 5d, 04103 Leipzig, Germany. Status: 01/2023
The company brochure gives an overview of the focus and competences of CO.DON GmbH.